Volume 9, Issue 3 (12-2023)
J Sport Biomech 2023, 9(3): 234-250 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azadian E, Majlesi M, Fatahi A, Bakhtiyarian R. Evaluation of Spatio-Temporal Gait Variability during Obstacle Crossing in Parkinson's Disease. J Sport Biomech 2023; 9 (3) :234-250
URL: http://biomechanics.iauh.ac.ir/article-1-328-en.html
URL: http://biomechanics.iauh.ac.ir/article-1-328-en.html
1- Department of Motor Behavior, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
2- , Department of Sport Biomechanics, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
3- Department of Sports Biomechanics, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
4- Department of Sport Biomechanics, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
2- , Department of Sport Biomechanics, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
3- Department of Sports Biomechanics, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
4- Department of Sport Biomechanics, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
Full-Text [PDF 2121 kb]
(697 Downloads)
| Abstract (HTML) (2451 Views)
Full-Text: (1321 Views)
Extended Abstract
1. Introduction
Parkinson's disease (PD) is the second most progressive and debilitating age-related neurological disorder worldwide, with its prevalence increasing in individuals over 60 years old. In this disease, a wide spectrum of central physiological processes affecting posture control and balance is influenced. For example, decreased muscle strength and function, reduced cognitive function, history of previous falls, and fear of recurrent falls are among the factors that increase the risk of falling. Negotiating obstacles and complex environments is a challenging task in daily life and is recognized as the leading cause of falls in the elderly and individuals with PD. Successful negotiation requires planning and visual guidance to adjust steps, occurring at least 6 steps before encountering the obstacle. Walking adaptations include changing walking speed and increasing step-to-step variability as approaching the obstacle, which increases with age. Previous studies have shown that due to increased motor and sensory impairments associated with aging, elderly individuals tend to increase step height and decrease walking speed compared to younger individuals when negotiating obstacles. Studies have shown that Parkinson's patients who experience freezing of gait (FOG), even when in the ON medication state, demonstrate greater variability in spatial-temporal parameters of walking compared to patients who do not experience FOG. This study aimed to investigate the effect of negotiating obstacles on spatial-temporal parameters and the variability of these parameters in individuals with Parkinson's disease compared to neurologically healthy counterparts.
2. Methods
The participants of this study included elderly individuals residing in the Hamedan province. A total of 32 elderly individuals were selected using convenience sampling. Fifteen patients with Parkinson's disease (PD) were recruited from the specialized neurology clinics. Additionally, 17 healthy elderly individuals were selected as the control group. Motion analysis was performed using a three-dimensional Vicon motion analysis system (Vicon Peak, Oxford, UK) with four T20 series cameras at a frequency of 100 Hz. Spherical markers with a diameter of 14 mm were attached to specific anatomical points on the lower limbs of the participants using double-sided adhesive tape and based on the Plug-In Gait Marker Set model (Vicon Peak, Oxford, UK). Two Kistler force plates (Kistler 9281EA, Winterthur, Switzerland) with a sampling frequency of 1000 Hz synchronized with the cameras were used to determine various gait events in different tasks. Participants walked barefoot at a normal speed and walked while crossing obstacles. The obstacle, made of flexible foam plastic, had dimensions of 60 cm length, 6 cm diameter, and 15 cm height. It was designed to be placed between two force plates so that there was no contact with the force plate surface during obstacle crossing. For the analysis of spatial-temporal gait parameters, a three-way analysis of variance (ANOVA) was employed. The factors examined for variability and mean spatial-temporal parameters included group (PD and control groups), task (normal walking and walking with obstacle), and the interaction between these factors. All statistical analyses were conducted using SPSS software (SPSS 16, SPSS Inc., Chicago, IL, USA), with a significance level of p < 0.05.
3. Results
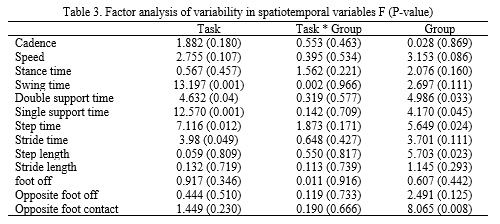
Factor analysis results indicated that in the PD group, cadence (approximately 20%) and gait velocity (approximately 31%) were significantly lower compared to the control group. Meanwhile, swing time (approximately 16%), stance time (approximately 24%), stride time (approximately 22%), step time (approximately 24%), single stance time (approximately 16%), and double stance time (approximately 38%) were significantly higher in the PD group compared to the control group. The results regarding task factor showed that this factor had a significant effect on most spatial-temporal variables. Factor analysis results also showed that the variability in all spatial-temporal gait variables was higher in the PD group compared to the control group. However, in variables such as double stance time (approximately 58%), single stance time (approximately 31%), stride time (approximately 70%), step length (approximately 43%), and percentage of foot contact with the ground (approximately 53%), it was significantly higher in the PD group compared to the control group (Table 3). The obstacle factor also showed a significant effect on the variability of some parameters. Crossing the obstacle compared to normal walking in both groups led to a significant increase in variability in swing time (approximately 71%), single stance time (approximately 57%), stride time (approximately 35%), and step time (approximately 36%), and only variability in double stance time (approximately 36%) decreased during obstacle crossing. The interaction between group and task in these variables was not significant, indicating that the obstacle factor increased variability in both groups (Table 3).
4. Conclusion
The aim of this study was to investigate the impact of obstacle crossing on spatial-temporal gait parameters and to examine the variability of these parameters. The results of a correlation study were consistent with the findings of the current study, indicating that for the PD group, the risk of falling was strongly associated with certain gait parameters such as speed, stride length, and step time. In contrast, for healthy elderly individuals, the risk of falling was associated with balance metrics such as path length and sway in the AP and ML directions. Interestingly, there was no strong relationship between the risk of falling and balance metrics for individuals with PD, despite showing greater oscillation compared to healthy elderly individuals. Obstacle crossing also resulted in a significant increase in step time, stride time, step length, and stride length compared to normal walking. However, percentage variables during obstacle crossing showed a significant decrease, which was more pronounced in the PD group. In previous research, the most important temporal factors in gait are the stance and swing phases. The stance phase accounts for approximately 60% of the gait cycle, with the remaining 40% attributed to the swing phase. According to Schmidt's motor program theory, the temporal ratio of these two variables remains constant when the gait pattern is consistent, such as walking at different speeds. However, the results of this study showed that obstacle crossing resulted in a significant change in the timing between the stance and swing phases. In other words, the ratio of these phases in normal walking and walking during obstacle crossing had a significant difference. Therefore, it can be concluded that the task of obstacle crossing is controlled by a different motor program.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be addressed in this research.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to preparing the article.
Conflicts of interest
The authors declared no conflict of interest.
Parkinson's disease (PD) is the second most progressive and debilitating age-related neurological disorder worldwide, with its prevalence increasing in individuals over 60 years old. In this disease, a wide spectrum of central physiological processes affecting posture control and balance is influenced. For example, decreased muscle strength and function, reduced cognitive function, history of previous falls, and fear of recurrent falls are among the factors that increase the risk of falling. Negotiating obstacles and complex environments is a challenging task in daily life and is recognized as the leading cause of falls in the elderly and individuals with PD. Successful negotiation requires planning and visual guidance to adjust steps, occurring at least 6 steps before encountering the obstacle. Walking adaptations include changing walking speed and increasing step-to-step variability as approaching the obstacle, which increases with age. Previous studies have shown that due to increased motor and sensory impairments associated with aging, elderly individuals tend to increase step height and decrease walking speed compared to younger individuals when negotiating obstacles. Studies have shown that Parkinson's patients who experience freezing of gait (FOG), even when in the ON medication state, demonstrate greater variability in spatial-temporal parameters of walking compared to patients who do not experience FOG. This study aimed to investigate the effect of negotiating obstacles on spatial-temporal parameters and the variability of these parameters in individuals with Parkinson's disease compared to neurologically healthy counterparts.
2. Methods
The participants of this study included elderly individuals residing in the Hamedan province. A total of 32 elderly individuals were selected using convenience sampling. Fifteen patients with Parkinson's disease (PD) were recruited from the specialized neurology clinics. Additionally, 17 healthy elderly individuals were selected as the control group. Motion analysis was performed using a three-dimensional Vicon motion analysis system (Vicon Peak, Oxford, UK) with four T20 series cameras at a frequency of 100 Hz. Spherical markers with a diameter of 14 mm were attached to specific anatomical points on the lower limbs of the participants using double-sided adhesive tape and based on the Plug-In Gait Marker Set model (Vicon Peak, Oxford, UK). Two Kistler force plates (Kistler 9281EA, Winterthur, Switzerland) with a sampling frequency of 1000 Hz synchronized with the cameras were used to determine various gait events in different tasks. Participants walked barefoot at a normal speed and walked while crossing obstacles. The obstacle, made of flexible foam plastic, had dimensions of 60 cm length, 6 cm diameter, and 15 cm height. It was designed to be placed between two force plates so that there was no contact with the force plate surface during obstacle crossing. For the analysis of spatial-temporal gait parameters, a three-way analysis of variance (ANOVA) was employed. The factors examined for variability and mean spatial-temporal parameters included group (PD and control groups), task (normal walking and walking with obstacle), and the interaction between these factors. All statistical analyses were conducted using SPSS software (SPSS 16, SPSS Inc., Chicago, IL, USA), with a significance level of p < 0.05.
3. Results
Factor analysis results indicated that in the PD group, cadence (approximately 20%) and gait velocity (approximately 31%) were significantly lower compared to the control group. Meanwhile, swing time (approximately 16%), stance time (approximately 24%), stride time (approximately 22%), step time (approximately 24%), single stance time (approximately 16%), and double stance time (approximately 38%) were significantly higher in the PD group compared to the control group. The results regarding task factor showed that this factor had a significant effect on most spatial-temporal variables. Factor analysis results also showed that the variability in all spatial-temporal gait variables was higher in the PD group compared to the control group. However, in variables such as double stance time (approximately 58%), single stance time (approximately 31%), stride time (approximately 70%), step length (approximately 43%), and percentage of foot contact with the ground (approximately 53%), it was significantly higher in the PD group compared to the control group (Table 3). The obstacle factor also showed a significant effect on the variability of some parameters. Crossing the obstacle compared to normal walking in both groups led to a significant increase in variability in swing time (approximately 71%), single stance time (approximately 57%), stride time (approximately 35%), and step time (approximately 36%), and only variability in double stance time (approximately 36%) decreased during obstacle crossing. The interaction between group and task in these variables was not significant, indicating that the obstacle factor increased variability in both groups (Table 3).

4. Conclusion
The aim of this study was to investigate the impact of obstacle crossing on spatial-temporal gait parameters and to examine the variability of these parameters. The results of a correlation study were consistent with the findings of the current study, indicating that for the PD group, the risk of falling was strongly associated with certain gait parameters such as speed, stride length, and step time. In contrast, for healthy elderly individuals, the risk of falling was associated with balance metrics such as path length and sway in the AP and ML directions. Interestingly, there was no strong relationship between the risk of falling and balance metrics for individuals with PD, despite showing greater oscillation compared to healthy elderly individuals. Obstacle crossing also resulted in a significant increase in step time, stride time, step length, and stride length compared to normal walking. However, percentage variables during obstacle crossing showed a significant decrease, which was more pronounced in the PD group. In previous research, the most important temporal factors in gait are the stance and swing phases. The stance phase accounts for approximately 60% of the gait cycle, with the remaining 40% attributed to the swing phase. According to Schmidt's motor program theory, the temporal ratio of these two variables remains constant when the gait pattern is consistent, such as walking at different speeds. However, the results of this study showed that obstacle crossing resulted in a significant change in the timing between the stance and swing phases. In other words, the ratio of these phases in normal walking and walking during obstacle crossing had a significant difference. Therefore, it can be concluded that the task of obstacle crossing is controlled by a different motor program.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be addressed in this research.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to preparing the article.
Conflicts of interest
The authors declared no conflict of interest.
Type of Study: Research |
Subject:
Special
Received: 2024/02/2 | Accepted: 2024/02/18 | Published: 2024/02/19
Received: 2024/02/2 | Accepted: 2024/02/18 | Published: 2024/02/19
References
1. Morrison S, Moxey J, Reilly N, Russell DM, Thomas KM, Grunsfeld AA. The relation between falls risk and movement variability in Parkinson's disease. Experimental brain research. 2021;239(7):2077-87. [DOI:10.1007/s00221-021-06113-9] [PMID]
2. Siragy T, Nantel J. Quantifying dynamic balance in young, elderly and Parkinson's individuals: a systematic review. Frontiers in aging neuroscience. 2018;10:387. [DOI:10.3389/fnagi.2018.00387] [PMID]
3. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease (Primer). Nature Reviews: Disease Primers. 2017;3(1). [DOI:10.1038/nrdp.2017.13] [PMID]
4. Dennison AC, Noorigian JV, Robinson KM, Fisman DN, Cianci HJ, Moberg P, et al. Falling in Parkinson disease: identifying and prioritizing risk factors in recurrent fallers. American journal of physical medicine & rehabilitation. 2007;86(8):621-32. [DOI:10.1097/PHM.0b013e311611583] [PMID]
5. Perera T, Tan JL, Cole MH, Yohanandan SA, Silberstein P, Cook R, et al. Balance control systems in Parkinson's disease and the impact of pedunculopontine area stimulation. Brain. 2018;141(10):3009-22. [DOI:10.1093/brain/awy216] [PMID]
6. Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson's disease: a complex and evolving picture. Movement disorders. 2017;32(11):1524-36. [DOI:10.1002/mds.27195] [PMID]
7. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn P. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116-24. [DOI:10.1212/WNL.0b013e3181e7b688] [PMID]
8. Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson's disease: a review of fall risk factors and the role of physical interventions. Neurodegenerative disease management. 2014;4(3):203-21. [DOI:10.2217/nmt.14.22] [PMID]
9. Hausdorff JM, Zitser J, Mirelman A, Giladi N. Interaction between cognition and gait in patients with Parkinson's disease. Cognitive impairment and dementia in Parkinson's disease: Oxford University Press London; 2010. 87-100. [DOI:10.1093/med/9780199564118.003.008] [PMID]
10. Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride‐to‐stride variations of gait cycle timing in Parkinson's disease and Huntington's disease. Movement disorders. 1998;13(3):428-37. [DOI:10.1002/mds.870130310] [PMID]
11. Ma L, Mi T-M, Jia Q, Han C, Chhetri JK, Chan P. Gait variability is sensitive to detect Parkinson's disease patients at high fall risk. International Journal of Neuroscience. 2022;132(9):888-93. [DOI:10.1080/00207454.2020.1849189] [PMID]
12. Nanhoe-Mahabier W, Snijders A, Delval A, Weerdesteyn V, Duysens J, Overeem S, et al. Walking patterns in Parkinson's disease with and without freezing of gait. Neuroscience. 2011;182:217-24. [DOI:10.1016/j.neuroscience.2011.02.061] [PMID]
13. Nanhoe-Mahabier W, Snijders A, Delval A, Weerdesteyn V, Duysens J, Overeem S, et al. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience. 2013;236:110-6. [DOI:10.1016/j.neuroscience.2013.01.038] [PMID]
14. Simieli L, Barbieri FA, Orcioli-Silva D, Lirani-Silva E, Beretta VS, Santos PCRd, et al. Variability of crossing phase in older people with Parkinson's disease is dependent of obstacle height. Scientific Reports. 2018;8(1):14852. [DOI:10.1038/s41598-018-33312-2] [PMID]
15. Gérin-Lajoie M, Richards CL, McFadyen BJ. The circumvention of obstacles during walking in different environmental contexts: a comparison between older and younger adults. Gait & posture. 2006;24(3):364-9. [DOI:10.1016/j.gaitpost.2005.11.001] [PMID]
16. Patla AE, Greig M. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neuroscience letters. 2006;397(1-2):110-4. [DOI:10.1016/j.neulet.2005.12.016] [PMID]
17. Lythgo N, Begg R, Best R. Stepping responses made by elderly and young female adults to approach and accommodate known surface height changes. Gait & posture. 2007;26(1):82-9. [DOI:10.1016/j.gaitpost.2006.07.006] [PMID]
18. Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson's disease. Gait & posture. 2011;34(3):329-33. [DOI:10.1016/j.gaitpost.2011.05.020] [PMID]
19. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Movement disorders: official journal of the Movement Disorder Society. 2004;19(8):871-84. [DOI:10.1002/mds.20115] [PMID]
20. Hausdorff J, Schaafsma J, Balash Y, Bartels A, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Experimental brain research. 2003;149:187-94. [DOI:10.1007/s00221-002-1354-8] [PMID]
21. Simieli L, Gobbi LTB, Orcioli-Silva D, Beretta VS, Santos PCR, Baptista AM, et al. The variability of the steps preceding obstacle avoidance (approach phase) is dependent on the height of the obstacle in people with Parkinson's disease. Plos one. 2017;12(9):e0184134. [DOI:10.1371/journal.pone.0184134] [PMID]
22. Pieruccini-Faria F, Montero-Odasso M. Obstacle negotiation, gait variability, and risk of falling: Results from the "gait and brain study". The Journals of Gerontology: Series A. 2019;74(9):1422-8. [DOI:10.1093/gerona/gly254] [PMID]
23. Terrier P, Schutz Y. Variability of gait patterns during unconstrained walking assessed by satellite positioning (GPS). European journal of applied physiology. 2003;90:554-61. [DOI:10.1007/s00421-003-0906-3] [PMID]
24. Ferrari A, Benedetti MG, Pavan E, Frigo C, Bettinelli D, Rabuffetti M, et al. Quantitative comparison of five current protocols in gait analysis. Gait & posture. 2008;28(2):207-16. [DOI:10.1016/j.gaitpost.2007.11.009] [PMID]
25. Winter DA. Biomechanics and motor control of human movement: John Wiley & Sons; 2009. [DOI:10.1002/9780470549148]
26. Whittle MW. Gait analysis: an introduction: Butterworth-Heinemann; 2014.
27. Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement Disorders. 2006;21(7):950-7. [DOI:10.1002/mds.20848] [PMID]
28. Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(8):896-901. [DOI:10.1093/gerona/glp033] [PMID]
29. Richards J, Levine D, Whittle MW, editors. Whittle's Gait Analysis-E-Book. Elsevier Health Sciences; 2022 Aug 28.
30. Shapiro DC, Zernicke RF, Gregor RJ, Diestel JD. Evidence for generalized motor programs using gait pattern analysis. Journal of motor behavior. 1981;13(1):33-47. [DOI:10.1080/00222895.1981.10735235] [PMID]
31. Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Human movement science. 2007;26(4):555-89. [DOI:10.1016/j.humov.2007.05.003] [PMID]
32. Bryant MS, Rintala DH, Hou J-G, Collins RL, Protas EJ. Gait variability in P arkinson's disease: levodopa and walking direction. Acta Neurologica Scandinavica. 2016;134(1):83-6. [DOI:10.1111/ane.12505] [PMID]
33. Puyjarinet F, Bégel V, Gény C, Driss V, Cuartero M-C, Kotz SA, et al. Heightened orofacial, manual, and gait variability in Parkinson's disease results from a general rhythmic impairment. npj Parkinson's Disease. 2019;5(1):19. [DOI:10.1038/s41531-019-0092-6] [PMID]
34. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57(3):B115-B25. [DOI:10.1093/gerona/57.3.B115] [PMID]
35. Vitório R, Pieruccini-Faria F, Stella F, Gobbi S, Gobbi LTB. Effects of obstacle height on obstacle crossing in mild Parkinson's disease. Gait & posture. 2010;31(1):143-6. [DOI:10.1016/j.gaitpost.2009.09.011] [PMID]
36. Dirnberger G, Jahanshahi M. Executive dysfunction in P arkinson's disease: A review. Journal of neuropsychology. 2013;7(2):193-224. [DOI:10.1111/jnp.12028] [PMID]
37. Lo O-Y, van Donkelaar P, Chou L-S. Distracting visuospatial attention while approaching an obstacle reduces the toe-obstacle clearance. Experimental brain research. 2015;233:1137-44. [DOI:10.1007/s00221-014-4189-1] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








